6 Indispensable Tools To Drive Effective User-Centered Design in Med Device
Craig Scherer and Carolyn Rose talk about the importance of effectively identifying, prioritizing, and translating user needs into design guidance in their latest Med Device Online article
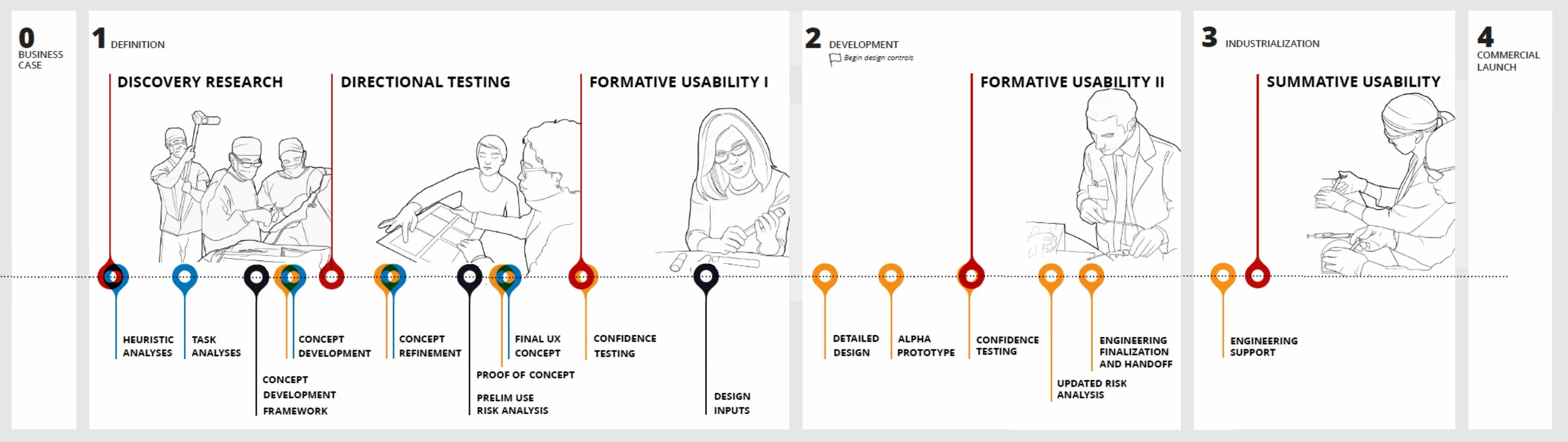

Turning user needs into design guidance is a key component of developing a successful medical device. The primary goal of this process is to develop a device that not only meets the FDA’s criteria for safety and effectiveness, but also one that provides the best possible user experience for all stakeholders.
The successful application of user-centered design principles relies on iterative and frequent feedback from stakeholders throughout the entire development process. This research also provides the design team information with which to identify, document, and better manage risk throughout the design cycle.
Learn more about these six tools in the full article on MedDevice Online.
For a deeper dive in our user research activities, check out our user needs poster.
