ivWatch
Lean development to ensure a startup’s success
Challenge

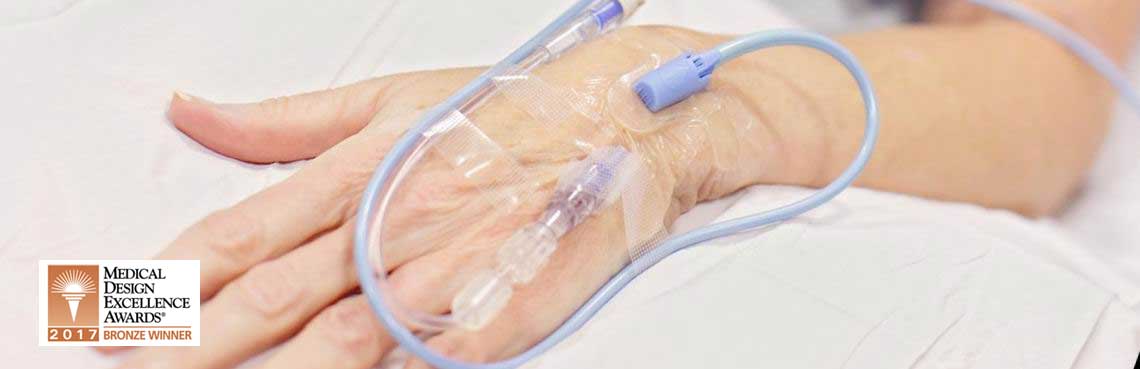
Millions of intravenous (IV) lines are set every year, but at least 23% of them experience some level of infiltration. This infiltration compromises the effectiveness of their drug delivery therapy, and in extreme cases, can lead to amputation or even death.
ivWatch LLC, a medical device startup headquartered in the Washington D.C. area, has developed a technology to sense these infiltrations at the source. With this core technology and a high level of clinical expertise, they sought the partnership of Insight to develop and industrialize its product potential. This startup’s goal was to stay lean while finding the most expedient and safest route to market.
Solution
Together, ivWatch and Insight developed a continuous monitoring solution to aid in the early detection of peripheral IV infiltration. This technology has been FDA approved and ensures accurate delivery of medication and reduce drug dosing errors in adults as well as pediatric patients, a population that often cannot self-advocate.
“Insight proved to be a very collaborative and flexible development resource. Our product benefited greatly from their skill in merging technical, user experience and commercial requirements into an integrated design for the Model 400. Since completion of the Model 400, we have re-engaged them multiple times for additional work to help define how the platform might grow and evolve.”
-Gary Warren, President and CEO of ivWatch
Approach
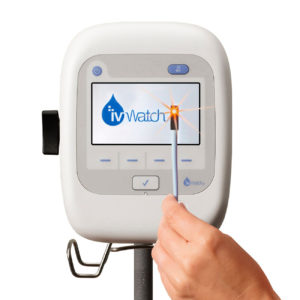
There is an interesting phenomenon in patient care regarding “preventative based technologies”. Care providers are most engaged when something goes wrong and treatment is compromised. With this insight, the continuous IV monitoring device is not seen as adding value when drug delivery is running smoothly. Therefore it is tantamount that the device is not perceived as adding complexity and steps but instead is easy to set up, use and interact with during normal cases. The efforts of the program had to focus on design, usability, and specifying a highly intuitive and easy to use interface.
Insight developed the device under design controls through to the industrialization phase, acting as the complete design and R&D department for this startup.