Combination Product Success Drivers
An ‘Insights’ interview with Mark Tunkel, Partner and Director of Business Development
We are here with Mark Tunkel to talk about developing effective and successful pharmaceutical delivery systems. Mark is partner at Insight and key account manager for many of Insight’s Pharma clients. Mark has been a significant contributor for the methods and processes that we bring to bear for this industry. So Mark…
How do you work with a client to develop a novel device vs those targeting a platform device? Are there significant differences?

“Well In both cases, it starts with the Patient Journey. We firmly believe a contextual understanding of the patient’s experience or patient discovery, is a very powerful tool when we employ it, especially in the earliest stages of a program. We find that it’s effective to support any drug delivery device strategy our clients might have, whether they’re branded or generic, and including any patient population from daily to less frequent use.”
Tell me more about the Patient Journey. How do you go about understanding and mapping it?
We use a technique called applied ethnography, which is a combination of interviews and in-context observations of practices, processes, and experiences within the patient’s home or any natural setting.
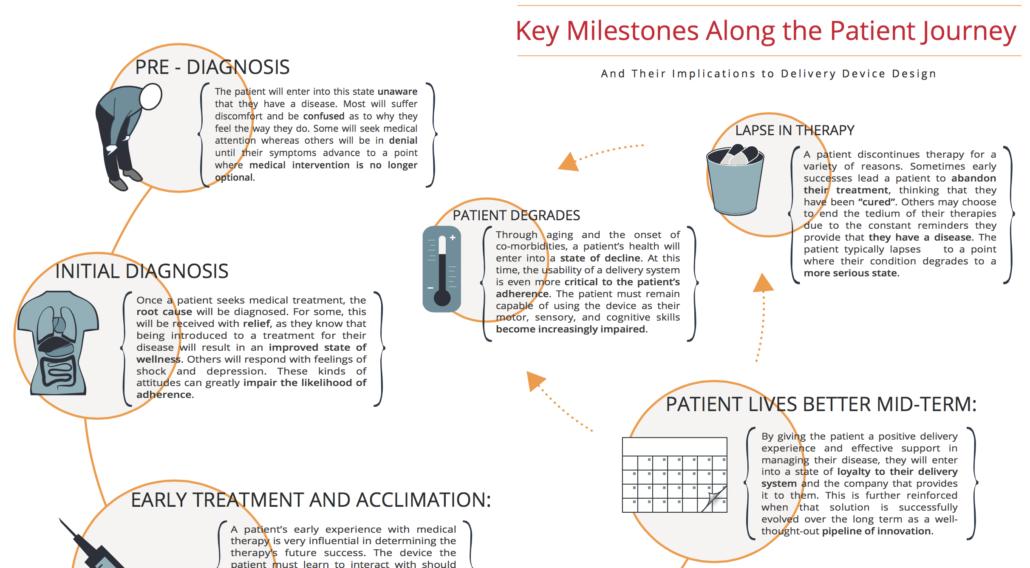
This allows us to gain a significant understanding of the environment, the social and emotional contexts, and all the other things that influence a patient’s use of a delivery system. At this early stage, we’re really looking at things broadly, potentially from when somebody receives their device, through the entire process of preparing, administering, and disposing of that particular device. We believe this gives us the most natural view of experience in use. We supplement this by gaining an understanding of the healthcare professional experience as well, as they are critically important in many times helping select a device for a specific patient population, and then certainly, training them as well.
Understanding both the patient and healthcare provider allows us to develop what we call journey maps, which demonstrates every bit of the process that somebody goes through in managing their disease; both from an administration standpoint and also from a longitudinal perspective over product life cycles and a patient’s progression with their disease.
These journey maps help us to:
- In the case of a novel device, understand and prioritize user needs to drive development of new to the world platform. It also allows us to help select the proper platform device and potentially customize it as well, if need be.
- Identify at a very early stage, potential for use errors or experiential gaps that we can address through the development of materials beyond the device such as instructions for use, novel training methods, or value-added packaging. We may uncover opportunities as well as longitudinal patient engagement that can be supported with connectivity and mobile applications.
- Provide an early indication of areas where risk might be present in clinical trials.

Ultimately, we use these processes and outputs to drive to development of innovative novel devices, or to selecting the right platform device. In either case, we have an understanding of where opportunities for differentiation might exist based on modifying a platform device for developing innovative features around a novel device, as well as training and longitudinal engagement.
What if the client has chosen a platform device? Does platform mean that everything is done and there is no other work required to complete development?
Platform devices provide many benefits, primarily in acceleration of product development and a proven supply chain, Intellectual property clearance, and for drug products that have relatively low patient populations or overall volume requirements, very attractive commercial terms, and significantly lower capital costs. That said, platform devices are designed to be broadly applicable to a potential set of patient types and drug product attributes. They are generally not developed to be specifically suited for a particular application.
As I mentioned earlier, we many times will develop an understanding of a patient Journey, or HCP preferences, to help guide our clients towards the appropriate platform device for a particular application, but that’s really only the beginning of the process.

We are commonly asked by clients who select a platform device if all of the human factors, risk management, characterization of performance relative to their drug product device verification, and so on, has been completed. This is really not the case. Ultimately, our clients are responsible for their own drug device combination. And the platform device, at that stage, is really only a set of components that are being provided by whoever their manufacturer is. It’s incumbent on the pharma company to ensure that this device, in combination with their drug, is safe and effective for the target population. This includes, as I mentioned, all human factors testing, risk management, and design verification.
There’s also the matter of differentiation, specifically in generics or biosimilars, where competitive companies may be targeting the same reference drug and device and may even be using the same platform device.
This is again where we can utilize the foundation of the patient journey to understand potential areas to differentiate things from device attributes and patient interface such as form, dosing windows, dial counters, grip architecture, and so on. Even if the actual device is fixed, We can also look at ways to add utility to packaging, instructions for use and quick start guides, and in training so that we can take advantage of the many benefits that come from platform devices, but then also find a way to drive some measure of differentiation.
So, while platform devices provide many benefits, they also require a lot of work to ensure that they are safe and effective for the target population, in that we are considering means of differentiation; both from a patient experience standpoint, but then also from a commercial operations perspective.
