
Congratulations to ivWatch, for their MDEA in the Nonsurgical Hospital Supplies and Equipment category.
Insight is proud to play have played a contributing factor in winning this prestigious award!
For More than 25 years, our user needs process has helped our clients discover, innovate, and develop new product solutions through a user-centered design approach. This most current award is further confirmation that considering user-centered design methodologies upfront is crucial toward the successful implementation and commercialization of medical devices today.
ivWatch
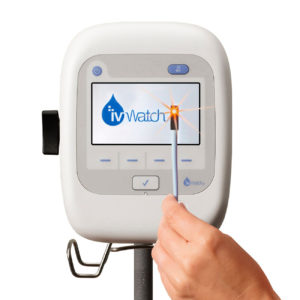
The ivWatch Model 400 is a medical device that continuously monitors peripheral IV sites to aid in the early detection of infiltrations. A sensor uses visible and near-infrared light to detect changes in optical properties of tissue. The device analyzes the data and notifies clinicians if conditions suggest an infiltration.
“Insight proved to be a very collaborative and flexible development resource. Our product benefited greatly from their skill in merging technical, user experience and commercial requirements into an integrated design for the Model 400. Since completion of the Model 400, we have re-engaged them multiple times for additional work to help define how the platform might grow and evolve.”
-Gary Warren, President and CEO of ivWatch
Congratulations again to ivWatch, and all of the 2017 MDEA winners. Winning a Medical Design Excellence Award is a great achievement, and Insight is thrilled to add #8 to our seven previous MDEA awards.
